On June 10th, I traveled to the south of Gombe to visit the range of the little-known Kalande community of chimpanzees.
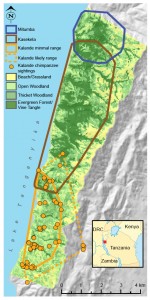
The Kalande community is one of three chimpanzee communities at Gombe.
The most famous, most intensively studied chimpanzees live in the Kasekela community in the center of the park. These are the chimpanzees that Jane Goodall has studied since 1960. They have the biggest range and the most members of the park’s three communities.
To the north of Kasekela live the Mitumba chimpanzees. This is a smaller community, which Deus Mjungu studied for his PhD research. The Mitumba community has fewer chimpanzees than Kasekela, but is still vigorous. They have a small range, but it includes excellent chimpanzee habitat with lots of food trees.
To the south is the Kalande community (also known as Bwavi). Most of the Kalande community’s range is grassland and woodland, with narrow strips of forest along the stream valleys. We know less about the Kalande chimpanzees than any of the others in Gombe. For the most part, these chimpanzees are still unhabituated, meaning they fear people. Researchers can follow the Kasekela and Mitumba chimpanzees around all day long, but they are lucky to get even fleeting glimpses of Kalande chimpanzees.
We aren’t even completely sure how many chimpanzees live in Kalande. Based on sightings and samples of genetically distinct individuals, there seem to be at least 9 chimpanzees in Kalande, but we don’t know for sure.
A small team of researchers monitor the Kalande chimpanzees. They collect fecal samples for genetic analysis, which enables us to keep track of individuals, even when we don’t know what they look like. Kalande has the highest rate of infection with the virus SIVcpz, which likely contributed to the decline of this community (Rudicell et al., 2010). Many females have left Kalande for other communities, both as part of the natural emigration process (females usually leave to join a new community when they are sexually mature), and because as Kalande declined, it eventually came to have too few males. Females seem to prefer living in communities with many males, both because many males are better able to defend the feeding territories that females need to survive and raise their offspring, and because females need unrelated males as mating partners. As the number of adult males in Kalande dropped down to one, or perhaps even zero, some Kalande females left for good, while others seem to have kept their Kalande home base, but visit Kasekela for mating.

Kati, for example, is a Kalande resident who has probably lived there since 1998. Based on genetic data, we think she is the daughter of Patti who was known as Tita when she was younger. Since 2006, Kati has been making occasional visits to Kasekela. I saw her with her young son Kazi on one of these early visits. Of the Kalande chimps, Kati seems to fear people the least, which would make since if she grew up in Kasekela.
Deus and I took the boat to Kalande, where we met Ashaabu, one of the new Kalande research assistants. Ashaabu got his start working as a village Forest Monitor for his village’s forest reserve (part of the Greater Gombe Ecosystem project). Before going into the forest, we talked with Ashaabu for a while about which chimpanzees he has been seeing.
Kazi, who was just a little boy back in 2006, now seems to be the alpha male of Kalande, even though he is just a gawky adolescent. Based on how old Kazi looked back in 2006, I think he must be at least 12 years old now. Ashaabu says Kazi is around the size of the Kasekela male Fundi, who is about 14. The old male Renadi (or Leonard) hasn’t been seen for a number of years now, and I suppose must be dead. There might be another adolescent male, Pamera, but we don’t know for sure if he is still alive. Ashaabu has regularly seen Kati, Kazai, Katarina (Kati’s new baby), a big female without an infant, an adolescent female (who I think might be Pairott), and another young female around Kazi’s size. (Perhaps this is really Pamera? Might be hard to tell he’s a male if he’s still young and seen only briefly from a distance.) Ashaabu also mentioned Obedina, a female who had a big belly last year who might also have a new baby now.
After talking, we hiked into the forest, climbing a steep rocky path into Nyamagoma valley. Nyamagoma is the southernmost valley of the park, just north of Kazinga village. The path wound through an open woodland with a view of the lake below.

Along the way, we collected fruits and leaves for the isotope and nutrition projects. Given that Kalande has so much woodland, it will be interesting to see if the Kalande chimpanzees, or their foods, differ isotopically at all from those in Kasekela.

We followed the path down towards Nyamagoma Stream, where tall trees grew, shading the steep valley in green light. We didn’t see any chimpanzees, but we did see a number of nests. Chimpanzees build a new nest (or bed) in trees each night to sleep safely out of reach of any predators that might be lurking about. We found one cluster with five fresh nests, suggesting that up to seven chimpanzees might have slept there (if the group included Kati and Obedina and their new babies). It was encouraging to see so many fresh signs of chimpanzees using this valley. The Kalande community is still hanging in there, and perhaps they might recover, if the Kasekela males don’t catch Kazi and finish him off.

Ashaabu carried with him the tablet computer he had used as a Forest Monitor. He used the tablet to take pictures of the nests and enter the data, including GPS locations of the nests. It was quite stunning for me to think each of the villages around Gombe now has its own Forest Monitors, collecting data like this on their own village forest reserves, and loading it up regularly into the Cloud.
Director of Chimpanzee Research for Gombe Stream Research Centre.
,