I grew up speaking English, first in Minnesota, then in central Illinois. On visits back to Minnesota, friends teased me for having a “southern” accent, like when I described a gar fish as looking “right like an alligator.” I encountered other varieties of English when we moved to Indiana — twangier than the downstate Illinois drawl — and when I went to college in Chicago. But these varieties of American English paled in comparison to what I encountered in Britain, when I spent a year as an exchange student at Cambridge University. As a fan of British television shows (Monty Python, Doctor Who, David Attenborough) and music (the Beatles, Pink Floyd), I expected to understand what people were saying. It turned out to be much trickier than I expected. Instead of the Received Pronunciation of BBC broadcasts, I encountered rapid speech with unfamiliar slang (“naff,” “grotty,” “phoar!”), new vocabulary words (“bog,” “crisps,” “snog”), expressions (“over the top,” “get off with,” “taking the mickey”), with consonants often ghosted as glottal stops. And there wasn’t just one variety to learn. People from different cities, regions, islands and social classes all spoke differently. Chris from Birmingham (“Brum”) spoke in a rapid series of unintelligible words and knowing looks that often left me completely baffled.
Different varieties of languages – dialects – likely emerge as an inevitable consequence of cultural evolution. People learn their home language from their parents, but they don’t learn it exactly the same. They add new words and phrases and ways of speaking that they pick up from their friends, and in the modern world, other sources like books, television, and the internet. As these differences accumulate, ways of speaking diverge. Descent with modification leads to the formation of new languages, just as in biology it produces new species.
Do other animals have dialects? Peter Marler, a pioneer in the study of animal behavior, demonstrated that some animals do. Songbirds have two main categories of calls: short, simple calls for everyday use, such as predator alarm calls, and more elaborate songs, mostly used during the mating season. Birds sing to defend territory, attract mates, and once mated, to communicate with their partner. Over the course of a long career, Marler conducted an elegant series of field studies and laboratory experiments, demonstrating that in species such as white-crowned sparrows, songs must be learned. A sparrow raised in isolation produces abnormal songs, but if he hears recordings of songs while growing up, he produces a normal song. Because sparrows learn their songs, local variants emerge and gradually change over time, producing regional dialects (Marler, 1970). (Here’s a video describing recent work on dialects in white-throated sparrows.)
Subsequent studies found evidence of such ability to modify calls — vocal learning — in some other species. Humpback whales, for example, sing long, complicated, haunting songs during their mating season. Like in sparrows, males do most of the singing. Whale songs vary not just by region, but also by year. In a given year in Hawaiian waters, male humpbacks sing songs that closely resemble one another, but which differ from what the whales are singing off the coast of Australia — and which also differ from last year’s favorite styles in Hawaii (Mercado et al., 2004).
Do species more closely related to humans, such as chimpanzees, show any evidence of vocal learning and dialects?
Marler pioneered the study of primate communication as well as birdsong. He visited Gombe National Park, Tanzania, in 1967, where he carried out the first modern field study of chimpanzee vocal communication. Based on his recordings from Gombe, Marler identified a set of 13 basic calls for chimpanzees. He also found that chimpanzee calls generally resembled those of gorillas (Marler, 1976). The similarity of calls among these apes suggests they are mainly under genetic control, rather than learned. However, John Mitani, working as a post-doctoral researcher with Marler, found some evidence for dialects in chimpanzee pant-hoot calls (Mitani et al., 1992).
When Jane Goodall gives public talks, she often begins by giving a pant-hoot: a loud call that begins with soft hoos, followed by grunts, barks or screams, and often ends with grunts again. (Jane usually leaves out the screams, though, as she thinks those sound too aggressive.)
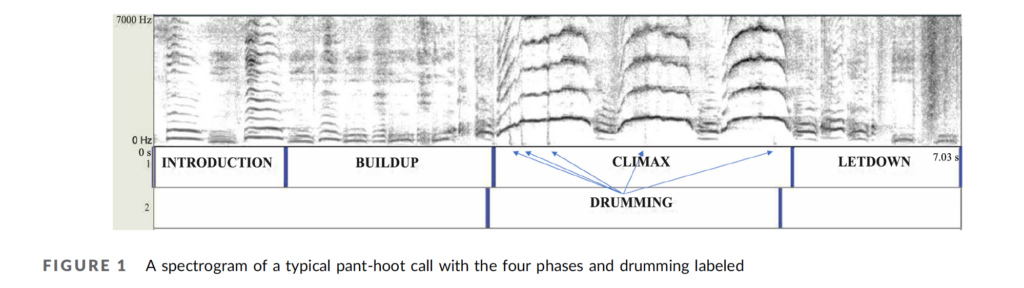
Pant-hoots are loud, and enable chimpanzees to communicate over long distances through the forest. Mitani recorded pant-hoots from chimpanzees at Mahale Mountains, some 160 km south of Gombe, and compared them to Marler’s recordings of pant-hoots from Gombe. Analysis revealed some subtle differences: Mahale chimpanzees produced shorter grunts at a faster rate than Gombe chimpanzees, and produced pant-hoots with higher-pitched screams (Mitani et al., 1992). Later studies reported similar geographic variation in calls among groups of captive chimpanzees (Marshall et al., 1999), between chimpanzees in Uganda and Tanzania (Arcadi et al., 1996), and among communities of chimpanzees at Taï Forest in Côte d’Ivoire (Crockford et al., 2004).
The idea that chimpanzees have distinct pant-hoot calls is an intriguing one. Moreover, the study led by Cathy Crockford (2004) reported that neighboring communities differed from one another more than they did from more distant communities. This suggested that chimpanzees produced calls that announced their community membership to other chimpanzees. This would make sense, given the hostile relations between chimp communities. A pant-hoot call would function like a sports team uniform or national flag, announcing the caller as a member of team Mitumba or Kasekela. Perhaps vocal learning evolved in our own ancestors in response to selection pressure from intergroup aggression?
As a graduate student, I conducted a series of playback experiments with chimpanzees in Kibale National Park, Uganda (Wilson et al., 2001). I used recordings that Mitani had made of chimpanzees from Mahale. In each experiment, I played back a single pant-hoot from a Mahale chimp from a speaker hidden hundreds of meters from Kibale chimpanzees. This single simulated intruder provoked an immediate and striking response. If the listening party consisted only of females, they silently climbed down from the tree where they had been resting or feeding and moved off in the opposite direction. If one or two males heard the call, they stayed silent, and either stayed in place, looking towards the source of the call, or slowly approached the speaker. If three or more males heard the call, though, they immediately gave a loud vocal response and rapidly approached the speaker, as if they were looking for an intruder to attack. So chimpanzees can clearly tell friend from foe by their voice. But were they responding to differences in dialect? Or just differences in familiar versus unfamiliar individuals?
As Mitani and his team continued to analyze calls from different chimpanzee sites, though, they dialed back on claims of dialects. When Mitani began recording calls at Kibale, he found that chimpanzees in Kibale do produce pant-hoots with build-up elements, despite the previous report that they don’t (Mitani et al., 1999). They also found that much of the variation in acoustic structure occurred within individuals, rather than between communities.
So, do chimpanzees have dialects or not? In an effort to answer this question, I worked with my graduate student, Nisarg Desai, and a team of Tanzanian field assistants to record and analyze chimpanzee vocalizations. Team members followed chimpanzees through the forest, carrying a hand-held “shotgun” microphone and a digital recorder, recording as many calls from each individual as possible.

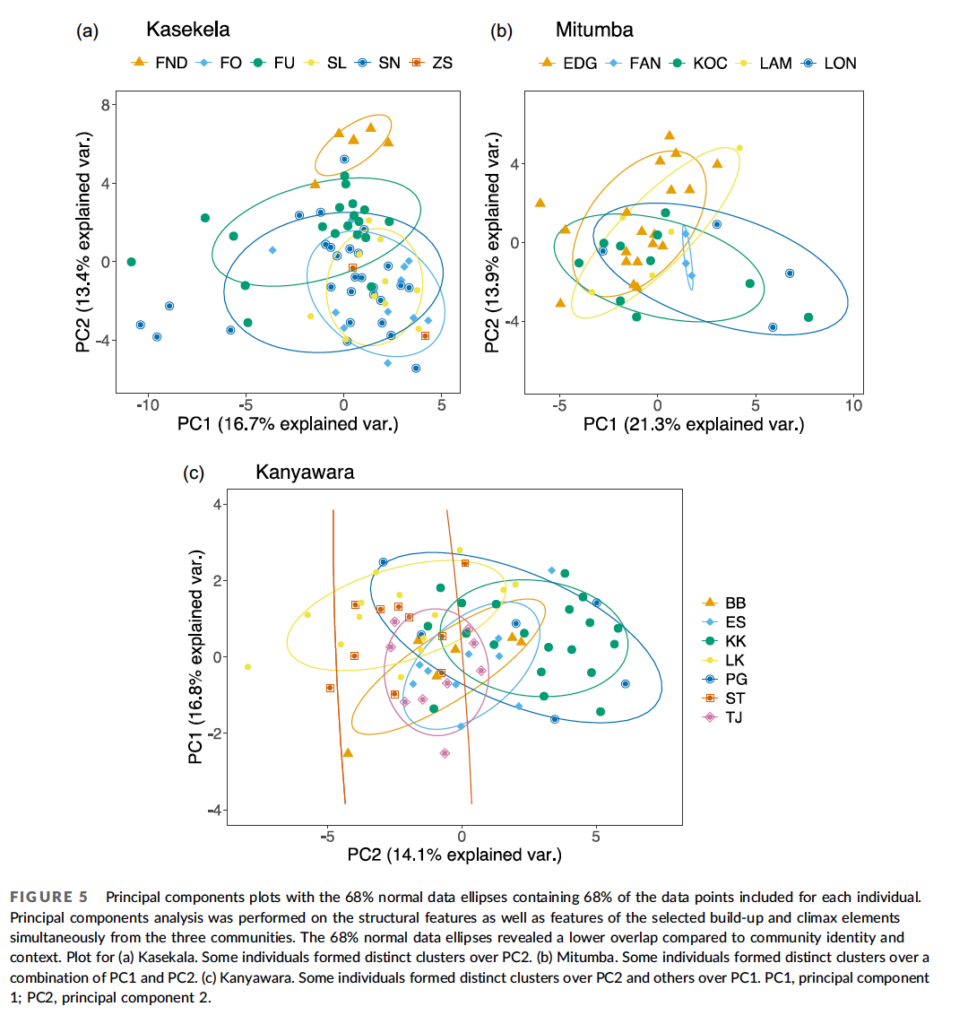
When Nisarg analyzed the data, he found that individuals varied a lot in the calls, but community membership explained little of the variation between the calls. Analyzing calls that Pawel Fedurek had recorded in Kibale, Nisarg again found lots of variation among individuals, but no clear geographical variation (Desai et al., 2022).
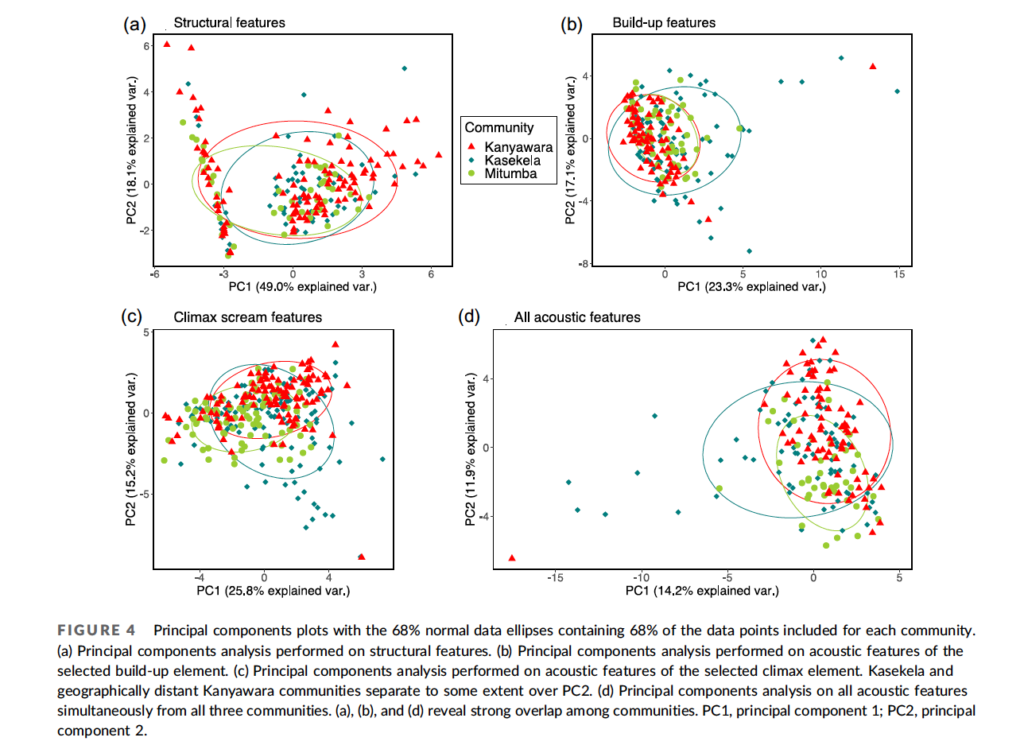
Based on Nisarg’s findings, and on the subtle differences reported from other studies, I’m inclined to think that chimpanzees do not have dialects after all. Chimpanzees and other apes may yet prove to have some limited capacity for vocal learning. But any such capacities in nonhuman apes pale in comparison, not just to humans, but also to many birds and whales in terms of vocal learning. This aspect of language seems to have emerged only after our ancestors diverged from the Pan-human ancestor.
All of this raises questions about what promotes vocal learning in other species, and whether similar factors promoted vocal learning in human ancestors. To be continued!

References
Arcadi, A. C. (1996). Phrase structure of wild chimpanzee pant hoots: patterns of production and interpopulation variability. American Journal of Primatology, 39(3), 159-178.
Crockford, C., Herbinger, I., Vigilant, L., & Boesch, C. (2004). Wild chimpanzees produce group‐specific calls: a case for vocal learning?. Ethology, 110(3), 221-243.
Desai, N. P., Fedurek, P., Slocombe, K. E., & Wilson, M. L. (2022). Chimpanzee pant‐hoots encode individual information more reliably than group differences. American Journal of Primatology, e23430.
Marler, P. (1970). Birdsong and speech development: Could there be parallels? There may be basic rules governing vocal learning to which many species conform, including man. American scientist, 58(6), 669-673.
Marler, P. (1976). Social organization, communication and graded signals: the chimpanzee and the. Growing Points Ethology, 239.
Marshall, A. J., Wrangham, R. W., & Arcadi, A. C. (1999). Does learning affect the structure of vocalizations in chimpanzees?. Animal behaviour, 58(4), 825-830.
Mercado, E., Herman, L.M. & Pack, A.A. Song copying by humpback whales: themes and variations. Anim Cogn 8, 93–102 (2005). https://doi.org/10.1007/s10071-004-0238-7
Mitani, J. C., Hasegawa, T., Gros‐Louis, J., Marler, P., & Byrne, R. (1992). Dialects in wild chimpanzees?. American Journal of Primatology, 27(4), 233-243.
Mitani, J. C., Hunley, K. L., & Murdoch, M. E. (1999). Geographic variation in the calls of wild chimpanzees: a reassessment. American Journal of Primatology: Official Journal of the American Society of Primatologists, 47(2), 133-151.
Wilson, M. L., Hauser, M. D., Wrangham, R. W. 2001. Does participation in intergroup conflict depend on numerical assessment, range location, or rank for wild chimpanzees? Animal Behaviour 61(6): 1203-1216. https://doi.org/10.1006/anbe.2000.1706